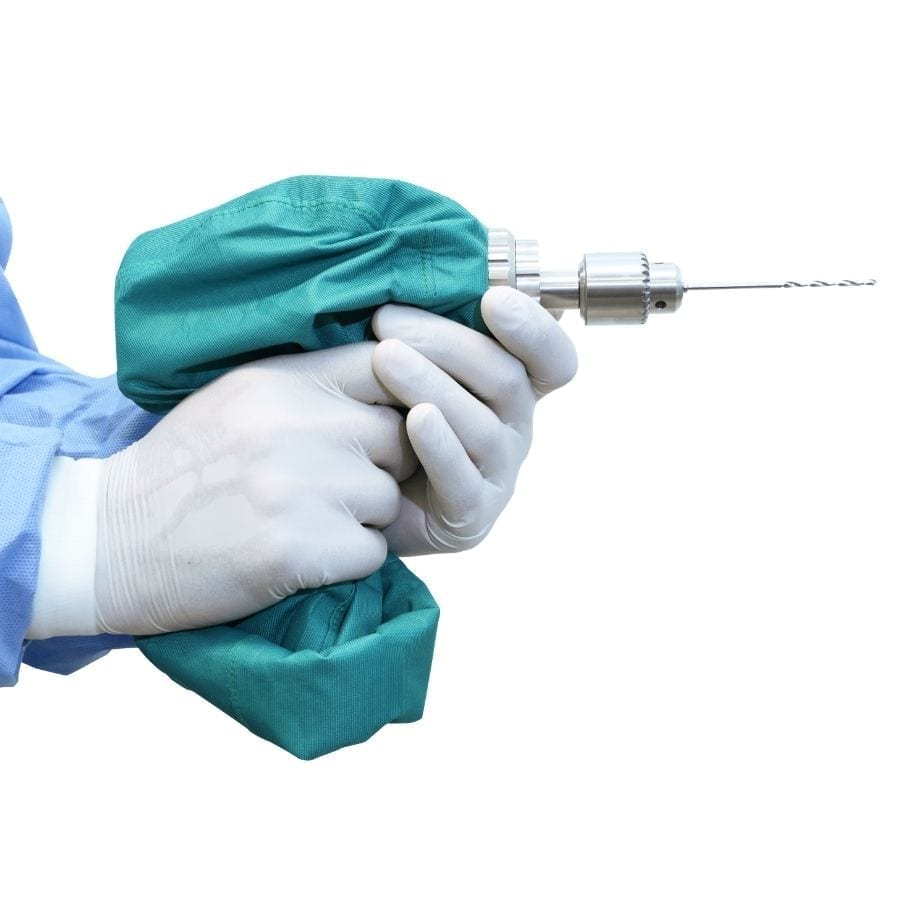
Key Features
Usability
Do more, faster. Conventional orthopaedic drills are expensive and one drill is not enough as it takes time to clean and sterilize instruments between cases. With the DrillCover Hex Starter Kit, multiple DrillCovers allow a busy hospital to perform back-to-back surgeries with a single drill by simply replacing the battery with a fully charged one to ensure plenty of power, replacing the 3-Jaw Chuck and loading the drill into a new sterile linen cover.
Design
This orthopaedic drill kit is designed to be highly ergonomic and easy to use. It is battery-powered, with 2 rechargeable Li-ion batteries included, and has a fully sealed liquid and pathogen proof sterile barrier.
Robust Durability
The DrillCover Hex Starter Kit features a robust and powerful drill with variable speed at 0 – 1050 RPM (rotations per minute) and drill stall torque of 70 in-Ibs (8 N-m). High friction/wear areas of the sterile cover are reinforced with a second layer of fabric for extra durability.
Specifications
Dewalt DCF610 Drill
- Speed: Variable at 0 – 1050 RPM (rotations per minute)
- Drill Stall Torque: 70 in-Ibs (8 N-m)
- Weight: 1 kg (2.2 Ibs)
- Sterilization: None required
DrillCover Hex Linen
- Material: Waterproof textile and construction (including fully-taped seams); pathogen-barrier, Level 4 ANSI/AAMI PB70 barrier performance against fluid penetration
- Lifetime: 75 uses
- Sterilization: Steam autoclave only
3-Jaw Chuck Adapter
- Material: Surgical-grade stainless steel
- Chuck Type: Keyed
- Capacity: 0.5mm – 7.4mm (0.020in – 0.291in)
Power
- Battery: 2x 12V rechargeable Lithium-ion batteries
- Battery Life: 30 min of continuous running
- Battery Charge Time: 30 min
- Charger Voltage & Plug Type: 120V, Plug Type A or or 240V, Plug Type C or G
Standard Accessories
- DrillCover Hex System Linen and Hardware Set which includes:
- Linen Cover (1x)
- 3-Jaw Chuck Adapter (1x)
- Chuck Key (1x)
Optional Accessories & Add-ons
- AO Small Quick Connect (RPA-009)
- Additional DrillCover Hex System Linen and Hardware Set
Product Documents, Manuals & Brochures

User Manual

Brochure

Specifications

Warranty
Regulatory Certificates

FDA Registration

ISO 13485

Health Canada Registration
Videos
FAQ
How is the DrillCover sterilized? Are all components sterilized?
All DrillCover System components can be sterilized by autoclave, except for the power tool, which remains non-sterile. Arbutus Medical instructs users to sterilize the linen and attachments using a gravity displacement autoclave (121°C, 30 mins exposure time, 60 min dry time) or a pre-vac autoclave (132°C, 4 min exposure time, 30 min dry time; or 134°C; 3 mins exposure time; 30 min dry time)
Is it safe to use a hardware drill for orthopaedic surgery?
Yes, but not with any drill. First, it is important to drill at speeds that avoid bone necrosis, and some hardware drills operate at maximum speeds 2-3x greater than standard orthopaedic drills, which therefore increases the risk of excessive heat generation at the drilling site.
Second, the drill must not interfere with other electronic equipment in the OR. Arbutus Medical has carefully selected and extensively tested both the DEWALT DCF610S2 (drill included in the DrillCover Hex System) and Makita DF032DZ (drill included in the DrillCover PRO System) to ensure complete safety.
Finally, the hardware drill must always be used with a sterile DrillCover System before it enters the sterile field. Before use, ensure your DrillCover System has been correctly assembled and that the textile is free of damage.
What is the difference between the DrillCover Hex and DrillCover PRO?
The DrillCover Hex includes only three components: a DrillCover linen, a 7.4mm chuck attachment (with chuck key), and a single-speed DEWALT 12V drill/driver (DCF610S2 – includes one charger and two batteries).
The DrillCover PRO has expanded capabilities: option to use multiple quick-connect attachments (including a keyless 7.4mm chuck and AO quick connect), a 2-speed drill (high-torque reaming mode, high-speed drilling mode), and the option to use a Cannulated or InLine adapter – all on a minimal footprint.
The DrillCover PRO is typically recommended if your team may have to perform the occasional definitive procedure, such as plating or intramedular nailing (requiring the DrillCover PRO reaming and/or cannulation features), or when your team requires maximum efficiency, in the event of a mass casualty scenario (requiring quick-change drill bits).
Do the DrillCovers ship sterile or non-sterile?
All DrillCovers ship non-sterile and must be sterilized before use.
Package Contents
- 1x DeWalt Drill
- 2x Rechargeable Li-ion batteries
- 1x Battery Charger (plug types A, C and G available)
- 1x DrillCover Hex Linen and Hardware Set (includes 1x DrillCover Linen, 1x 3-Jaw Chuck Adapter, and 1x Chuck Key)
Warranty
DewaltDCF610 Drill: Two (2) years from the original purchase date.
Consumable Linen: 90 days from original purchase date.
Chuck adapter: Two (2) years from the original purchase date.
Please see Product Resources box for a downloadable copy of Arbutus Medical warranty information.
Testimonials
“I am a trauma surgeon at Shock Trauma, and I oversaw the implementation of the Arbutus Medical’s DrillCover System within our Trauma Resuscitation Unit (TRU). Our hospital sees a high volume of trauma patients and the TRU is equipped to perform damage control procedures such as external fixation & skeletal traction which require the use of a power drill. We perform 5-20 such cases per week.
Formerly, the TRU did not have dedicated power and so residents were required to borrow power tools from the OR. This process was creating friction between the OR & TRU and was taking up the residents’valuable time.
The DrillCover system was purchased to provide dedicated power for the TRU. It is now 6 months after implementation, and we have found it has saved the residents’ time by reducing the need to borrow from the OR. It has proven to be a simple & cost effective solution for skeletal traction outside of the OR.”
June, 2020
Christopher LeBrun, MD
Assistant Clinical professor, Division of Orthopaedic Trauma, University of Maryland School of Medicine
Shock Trauma Center is a free-standing trauma hospital in Baltimore, Maryland and is part of the University of Maryland in the U.S.
“We had a very successful mission. 14 joint replacements and 1 pelvic/acetabular trauma case. We did that in 5 operative days. The tools worked exceptionally well and we were very pleased. Thank you for helping to make it successful! We were very blessed to have come across your company.”
Dr. Kelly Johnston MD, FRCSC
Chief, Division of Hip and Knee Reconstruction, Cumming School of Medicine, University of Calgary, Canada