Important Purchasing Information
Regulatory Restrictions : United States, Canada
7 days.
MOQ Shipping Dimensions (Depth), cm = 19.5
MOQ Shipping Dimensions (Length), cm = 65.5
MOQ Shipping Dimensions (Width), cm = 48.5
MOQ Shipping Weight (G.W) (kgs) = 20
Key Features
Design
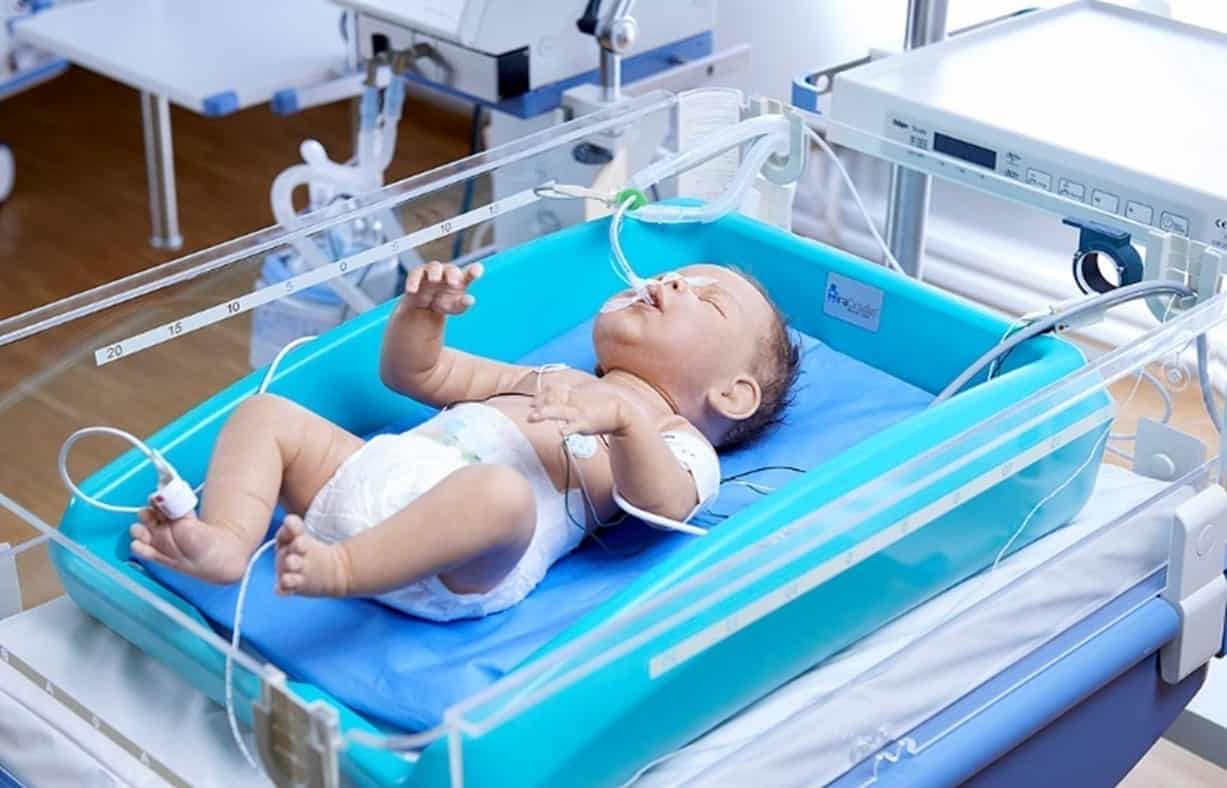
MiraCradle™ is efficient – it gives precise temperature control. It is also economical, costing less than 1/5th of the price of electronic cooling devices and is designed to be long-lasting for repeat use.
Usability
Minimal manual supervision required. PCMs can be charged in a normal refrigerator.
Safety
This is a cooling system that does not require an electrical supply near the baby to function, making this a safe cooling option.
Specifications
- Insulated Cradle: It is a rotomolded plastic structure which serves as a framework for placing all the other components of MiraCradle®- Neonate Cooler and also provides insulation to the PCM helping it last for longer hours
- savE® FS-29: This forms the bottom layer of the MiraCradle®-Neonate cooler. Three units of savE®FS-29 PCM are placed at the bottom of Cradle. savE®FS-29 in solid state passively extracts heat from the neonate’s body which is at 37°C thereby inducing and sustaining hypothermia
- savE® FS-21: This is the middle layer of the device. savE®FS-21 is used in conjunction with savE®FS-29 to quickly bring the temperature of the neonate down to 33°C. It is subsequently removed and savE® FS-29 takes over to sustain the temperature for longer hours
- Conduction Mattress: The conduction mattress is a gel bed which provides a smooth surface for the baby to lie on and improves heat transfer between the baby and the PCM
Standard Accessories
Optional Accessories & Add-ons
- Additional PCM savE® FS – 29
- Additional PCM savE® FS – 21
Product Documents, Manuals & Brochures

Brochure
Product Guide

Product Summary

User Manual
Assembly Guide
Neonatal HIE Pathophysiology Brochure

Therapeutic Hypothermia Risk Prevention Brochure

Therapeutic Hypothermia Mechanisms of Actions Brochure

Frequently Asked Questions
Regulatory Certificates

CE Certificate

ISO 13485 Certificate
Videos
Testimonials
– Dr. B.Vishnu Bhat
(Professor and Head, Department of Pediatrics, Division of Neonatology, JIPMER )
In India, asphyxia results in approximately quarter of the neonatal deaths and is a huge burden on neonatal morbidity and neurodevelopmental sequelae. Hypothermia is the only proven intervention to improve the neurodevelopmental outcomes; but because of the high cost of the currently available machines it is not available to most babies in the developing world. MiraCradle™ – Neonate Cooler is a significant low cost innovation to help such babies in this part of the world.
Dr. Sanjay Wazir MD, DM (Neonatology)
(Chief – Neonatology Medicine, Cloudnine Hospital, Gurgaon)
FAQ
What is birth asphyxia/ HIE?
It is a medical condition in which there is deficiency of oxygen in the baby’s body resulting in slow neurological development.
What is the intended use of MiraCradle® and how does it work?
MiraCradle® is used for inducing therapeutic hypothermia in babies suffering from birth asphyxia. It works by absorbing heat from the baby’s body to bring an equilibrium at desired temperature of 33.5°C for 72 hours. This is achieved using specifically engineered phase change materials (PCMs). The thermal energy storage technology and cascaded PCM design allow temperature to be maintained at a precise point throughout the course of therapy.
What is the recommended therapy?
Therapeutic hypothermia is the recommended therapy where the baby is cooled to temperatures 33°C-33.5°C for 72 hours; cooling reduces metabolism and therefore reduces the oxygen demand by brain cells preventing cell death.
When is the therapy given?
Asphyxiated babies should be given hypothermia within 6 hrs of birth. Since baby could have been asphyxiated even before birth, it is advisable to give hypothermia as soon as the possible.
Is there any additional equipment required when using the MiraCradle?
Yes. MiraCradle – Neonate Cooler induces and sustains hypothermia for 72 hours and provides precise temperature control. However, the following equipment used alongside the MiraCradle is essential for effective treatment of the newborn:
- Warmer: an infant radiant warmer is essential for using MiraCradle. The warming source is required to keep a check on temperature dropping below 33°C. The warmer should be used in manual mode, it is turned off when cooling is initiated and used when the temperature drops below the desired range.
- Neonatal rectal probe: required to monitor the core temperature of the newborn.
- Multi-parameter monitor: required for constant monitoring of the temperature and setting alarms for the desired temperature range of the newborn.
The treatment of HIE may also require other devices such as a ventilator, neuro-imaging devices, etc. The hospital and the doctor should have the required understanding and arrangements before starting the therapeutic hypothermia treatment.
What disinfectants can be used for cleaning the device?
For decontamination (contamination not due to soiling) of MiraCradle®, liquid chemicals are used to kill non-spore forming bacteria on the device. Some commonly used disinfectants are Sodium Hypochlorite (Bleach), ethyl alcohol, isopropyl alcohol (70%), Alconox, Liquinox, Cidex (Glutaraldehyde).
However, we recommend ethyl alcohol, isopropyl alcohol or mixture of two, with minimum concentration of 70%. Labels and stickers used on MiraCradle® are tested for compatibility with alcohols only. Any deviation from the recommendation is at the own risk of user.
I have more specific questions, where can I find the answers?
For more specific questions regarding assembly, use, cleaning, maintenance and repair, please consult the Frequently Asked Questions document available for download.
Package Contents
- 1x MiraCradle Main Unit – Insulated Cradle
- 6x savE® FS – 29
- 2x savE® FS – 21
- 1x Conduction mattress
- 1x Instruction boards
- 1x User manual
- 1x Registry for therapeutic hypothermia
Warranty
Insulated Cradle – 1 year warranty
savE® FS – 29 – 2 year warranty
savE® FS – 21 – 2 year warranty
Conduction Mattress – 1 year warranty