Buyers Guide
Newborn Resuscitation Devices
Why are Newborn Resuscitation Devices Important?
A large proportion of neonatal deaths can be prevented with the appropriate care and available commodities and products. Effective neonatal resuscitation includes immediate care with thorough drying, suction, stimulation, and positive-pressure ventilation in cases when the newborn is not breathing on their own. (2) Neonatal resuscitators are an essential medical device to save newborns from asphyxia at birth and one of 13 products included in the United Nations Commission on Life-Saving Commodities, given their role to end preventable deaths in newborns.
Types of newborn resuscitation devices- Suction and Positive Pressure Ventilation
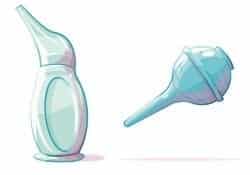
Manual Suction Bulbs are small, portable hand-held devices designed to suck gently and clear excessive mucus from the nose or mouth of a newborn to facilitate easier breathing, and are either single use (disposable) or multi-use).
Hand or foot-operated suction machines. In these devices, the vacuum effect is generated by human power, either by foot or by hand, but have the other components in common with electrical devices.
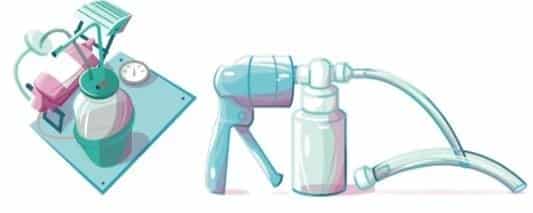
Electric suction machines consist of the power source (rechargeable battery and electric circuit), the vacuum source (pump) and the collection bottle (which may be a collection jar or disposable bag, with capacity of 1 L and be transparent), suction tubing, and vacuum gauge and regulator
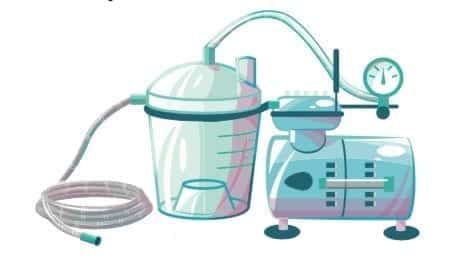
Devices for Positive Pressure Ventilation
Source: WHO Guidelines on Neonatal Resuscitation Devices
Key Considerations when Selecting Newborn Resuscitation Devices
- Suction safety. Safe ranges for neonatal suctioning depending on the size of the infant and are generally considered to be between 60-100mmHg.
- Bag with Mask Devices. Should have a bag size of 300-320L and masks of different sizes (0 for pre-term and low birth weight, and 1 for term infants) and a valve to maintain pressure or prevent exceeding a maximum pressure in range of 3kPa (30cmH20) -4.5 kPa (45 cmH20)
- Oxygen Source. Some T-piece devices may have an integrated blender, but if there are no oxygen sources available, the self-inflating bag may be most appropriate for settings that have very limited resources.
- Achieving Peak Inflation Pressure (PIP) and Positive End-Expiratory Pressure (PEEP) at 30/5 cm H20 with reducing percentage leak at the face mask with consistency and accuracy can be achieved with all 3 types, but may require different levels of training and practice. (4)
- Reprocessing and cleaning of reusable devices. When using reusable devices, it is important to adhere to infection prevention control (IPC) and manufacturing guidelines for pre-cleaning, disassembly, cleaning, sterilization, reassembly and storage to ensure clinical safety and duration of the device.
VIA Global Health is committed to supporting health systems access affordable and appropriate medical products to improve the health in their communities. Products included in our Buyers Guides are available for purchase at VIA Global Health.
References and Acknowledgments
(1) World Health Organization. Guidelines on Newborn Resuscitation, 2012. Found here
(2) World Health Organization. Technical Specifications of Neonatal Resuscitation Devices, 2016. Found here.
(3) Helping Babies Breathe 2nd Edition, American Academy of Pediatrics, 2016. found here
(4) Dawson J., Gerber et al. Providing PEEP during neonatal resuscitation: Which device is best? Journal of Pediatrics and Child Health. Volume 47, Issue 10. October 2011.
